Promising breakthrough in the field of acute myeloid leukaemia (AML)
An exciting new early intervention technique has emerged that may lead to a reduction in the number of sufferers of one of the most aggressive forms of blood cancer: AML. Whilst still in the preclinical stage, the study from the Dana-Farber/Boston’s children hospital has shown that it is possible to target and eradicate pre-leukemic cells before they can develop into cancer. This is a thrilling discovery which researchers believe can be applied to those at risk of developing AML. But what is this form of cancer and why should we care?
Acute myeloid leukaemia
Acute myeloid leukaemia (AML) is an aggressive form of blood cancer that affects around 3,200 people in the UK. It stems from young white blood cells called monocytes or granulocytes which are found in the bone marrow which is where all new blood cells are produced.
Every blood cell has the same beginning; as a stem cell. Stem cells are undifferentiated cells that have the potential to develop into any cell type. From here, they can develop into myeloid stem cells which subsequently become white blood cells (monocytes and granulocytes). This is shown in the diagram below.

In acute myeloid leukaemia, it’s the myeloid cells that are cancerous. This results in an overproduction of monocytes and granulocytes as they grow and divide too fast. These cells are not fully developed and can be dysfunctional, building up in the blood and bone marrow.
Normal myeloid cells have an important role in fighting infections, preventing the spread of tissue damage and protecting the body against parasites. This means sufferers of AML are more vulnerable to infections due to dysfunctional white blood cells. Additionally, as the white blood cells overcrowd the bone marrow this means there’s less space for other types of blood cells leading to:
– abnormal bruising and bleeding caused by reduced platelets.
– anaemia, shortness of breath and fatigue caused by reduced red blood cells.
If left untreated, the leukemic cells rapidly spread to other areas of the body including the lymph nodes and spleen, causing death within a few weeks or months. The prospect of detecting pre leukemic cells and stopping them before they develop into something more serious is therefore a significant breakthrough in the cancer field.
The “double-hit”

Like many cancers, it is thought that AML requires at least two genetic “hits” to develop. The first hit is known as clonal hematopoiesis of indeterminate potential (CHIP). This results in clones which are harmless if they remain dormant. It is the second “hit” that causes the cells to become malignant and ultimately results in the development of the cancer, with a 0.5 to 1% risk per year of progression from CHIP to malignancy. This premalignant stage makes AML an appealing system for investigating preventative cancer treatments.
A previous Swedish study in 2015 found that the chances of these hits occurring increases with age, as 10% of people older than 65 years old have such mutations compared with 1% of people under the age of 50. This explains the higher prevalence of AML in older people, as more than 40% of new cases are in people aged 75 and over. However, it can also occur in children.
The study itself
The idea behind the research was to use a compound, VTP-50469, to eradicate preleukemic cells before they become established leukaemia cells. They used knock in mice containing two mutations. The first “hit” was in the DNA methyltransferase 3A gene (DNMT3A) which can be detected in the CHIP phase. The second “hit” was in the NPM1 gene, which is absent from the CHIP phase and only detected in differentiated cells. It was confirmed that if untreated these mice developed AML. However, researchers found that when the mice are given the compound VTP-50469 the premalignant leukaemic cells were eradicated and so no development of leukeamia occured. There were no obvious toxic side effects and no harm done to normal blood cells.
VTP-50469 treated mice survived for more than 9 months compared to the average of 5 months in untreated groups. Additionally, after 300 days when the VTP-50469 treated mice were sacrificed, there was no detection of NP1MC mutations in the cells of bone marrow, liver or spleen. The data therefore implies that eradicating preleukemic NPM1c cells can be achieved without negatively impacting normal blood cells.
VTP-50469
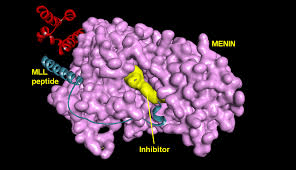
This compound has previously been used to treat established AML leukaemia. It acts by targeting two proteins: Menin and MLL. These two proteins join together and turn on genes that drive the leukaemia. This means when they are disrupted the complex falls apart and the leukaemia genes are turned off.
Wider implications
Whilst still in the preclinical phase, the future of preventative cancer treatment looks bright. This new research demonstrates a massive move in the right direction, being one of the first successful compounds that acts on preleukemic cells without having a toxic effect on normal cells. Though it would be impractical to screen everyone over a certain age for CHIP, screening and monitoring could be offered to those at increased risk of developing AML. This could include people who have been exposed to high levels of radiation, and those with a blood disorder or genetic condition such as down’s syndrome.
Original article link: https://science.sciencemag.org/content/367/6477/586